By David Martinelli, Ph.D.
Hearing depends on the transduction of sounds into neural signals by the inner hair cells of the cochlea. The cochlea also has outer hair cells with unique electromotile properties that increase auditory sensitivity, but they are particularly susceptible to damage by intense noise exposure and ototoxic drugs. Although the outer hair cells have synapses that allow communication with neurons that project to the brain, the function of this neuronal circuit and what auditory information it is sending to the brain is unclear. A previously proposed possible function for this circuit is that it may have a function in the detection of auditory pain, which would help us better understand hyperacusis, a condition in which a person experiences pain at a much lower volume level than listeners with typical hearing.
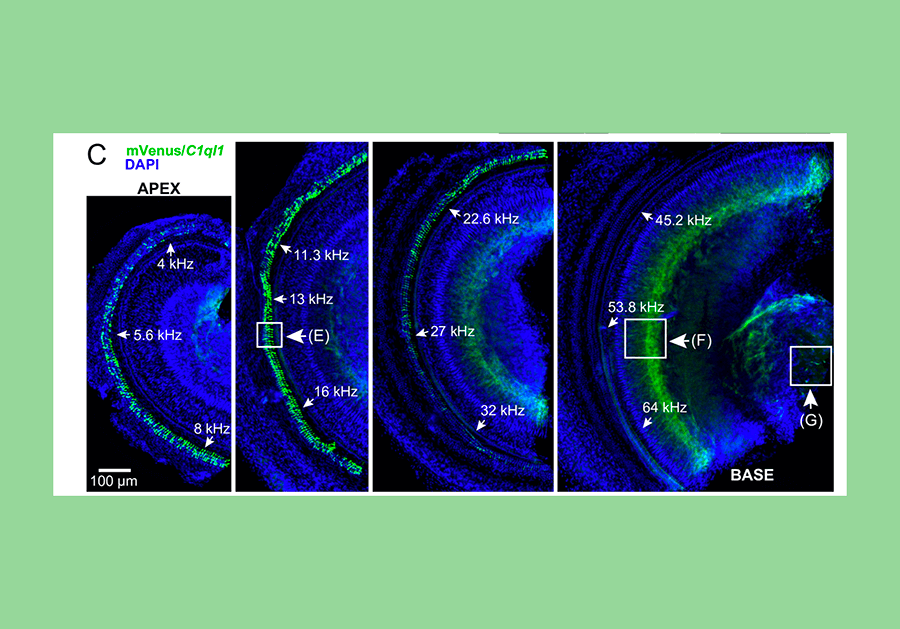
A novel strain of mice have been created such that a fluorescent protein called mVenus, which is similar to the jellyfish protein called green fluorescent protein, is made to be present inside any cell which expresses the gene C1ql1. In the mouse cochlea, this reveals that C1ql1 is expressed in the outer hair cells. A dye called DAPI stains each nucleus blue. Credit: David Martinelli, Ph.D.
As a postdoc, I had shown that the gene C1q-like 3 (C1ql3) and the C1QL3 protein it creates are important in allowing neurons to communicate with one another in the brain. Other research found that another gene in the same family, C1q-like 1 gene (C1ql1), is expressed specifically in outer hair cells, and not inner hair cells.
These findings prompted a new investigation into the possible functions of C1QL1 protein in the auditory system and a possible role at the synapses that may be contributing to the perception of auditory pain. We set out to investigate the function of C1QL1 in the cochlea using a mouse model where the gene is knocked out and no C1QL1 protein can be made. We hoped to find that removing this gene would provide evidence that the outer hair cell circuit to the brain contributes to the perception of auditory pain.
Surprisingly, as published in PLOS ONE in May 2021, we found C1QL1 expression in the cochlear tissue of adult mice, but not in neonatal or developing mice, indicating that the protein is not involved with the development of any aspect of the auditory system. This developmental regulation is surprising as both C1QL1 and the related C1QL3 have synaptogenic functions—the formation of synapses between neurons in the brain, especially during development—but C1QL1 expression is absent during the primary postnatal cochlear period of synaptogenesis. This suggests that the C1QL1 protein is not required for initial synaptogenesis in the cochlea. Correspondingly, we did not find that the absence of C1QL1 altered an animal’s apparent perception of auditory pain.
A promising future direction is to conduct similar experiments in older animals, as we found clues that the C1QL1 protein contributes to the maintenance of the outer hair cell synapses. Our research also alerts the larger cochlea scientific community about the expression of the C1ql1 gene specifically in outer hair cells, which may be useful for future experiments designed to better understand the function of these mysterious cells critical for hearing.
David Martinelli, Ph.D., is an assistant professor of neuroscience at the University of Connecticut Health Center. His 2019 Emerging Research Grant was generously funded by Hyperacusis Research Ltd.







Effortful listening is mentally, physically, and emotionally exhausting. Learn how it affects the brain—and what to do about it.