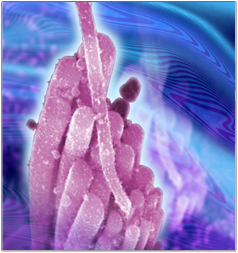
These findings reveal a previously unrecognized mechanism of hair cell regeneration with implications for how hair cells may be encouraged to regenerate in the mammalian inner ear.
Specific Group of Cochlear Cells in Mice Demonstrate Regenerative Potential
A surprising finding of this project was that a specific group of cells, called the greater epithelial ridge (GER), contained the majority of cells capable of growing into organoids. This ability can be interpreted as a form of regenerative potential because the GER cells can multiply and generate new sensory hair cells.
Uncovering a Signaling Molecule That Modulates Avian Hair Cell Regeneration
By Rebecca M. Lewis, Au.D., Ph.D., and Jennifer Stone, Ph.D.
Mammals including humans cannot regenerate hair cells, but other species such as birds and fish readily regenerate hair cells after damage to restore auditory function. The gene ATOH1 produces a protein that pushes supporting cells—cells that neighbor hair cells—to either directly convert into a hair cell or to divide and form a new hair cell. However, ATOH1 expression (when the gene is turned on) does not guarantee that hair cells develop in birds or mammals, which suggests that there are factors that prevent supporting cells from changing into hair cells. Identifying these factors in birds may help us better understand the lack of hair cell regeneration in mammals.
This schematic depicts our current ideas for how BMP4 regulates ATOH1 expression and therefore hair cell regeneration in the avian hearing organ. It shows (from left) typical hair cells, hair cell damage, and hair cell regeneration. Typical hair cells secrete BMP4. When hair cells die, BMP4 signaling is reduced, which allows ATOH1 to be expressed in supporting cells and pushes supporting cells to turn into hair cells. The newly regenerated hair cells secrete BMP4, suppressing ATOH1 in supporting cells and restoring the normal condition.
We examined the avian auditory system to characterize a potential inhibitor to ATOH1 during hair cell regeneration: bone morphogenetic protein 4 (BMP4). Bone morphogenetic proteins are secreted signaling molecules that regulate cellular processes in many regions of the body, including the nervous system. We found that BMP4 localizes to hair cells of the mature avian hearing organ and disappears when hair cells die or sustain damage. From this, we hypothesized that BMP4 may prevent ATOH1 expression in supporting cells and loss of BMP4 when hair cells die may enable ATOH1 to be expressed in supporting cells, driving them to convert into hair cells.
When we exposed avian auditory organs to BMP4 after selectively killing hair cells, this prevented ATOH1 expression and hair cell regeneration. When we antagonized BMP4 using an inhibitor, we found a generally opposite result: an increase in the number of regenerated hair cells.
We conclude that BMP4 is a potent inhibitor of ATOH1 and therefore suppresses hair cell regeneration. We recommend that BMP4 be explored further in studies of mammalian hair cell regeneration.
Published in Hearing Research on May 2, 2018, this study detailing BMP4’s negative effect on ATOH1 expands our knowledge of signaling molecules that suppress hair cell regeneration in birds and may also modulate hair cell regeneration in humans.
Rebecca M. Lewis, Au.D., Ph.D., is a clinical audiologist and auditory neuroscientist at Massachusetts Eye and Ear/Harvard Medical School in Boston. HRP researcher Jennifer Stone, Ph.D., is the director of research in the department of otolaryngology–head and neck surgery at the Virginia Merrill Bloedel Hearing Research Center at the University of Washington.
Empower the Hearing Restoration Project's life-changing research. If you are able, please make a contribution today.
Unraveling Genes Critical for Inner Ear Development
By Albert Edge, Ph.D., and Alain Dabdoub, Ph.D
The goal of the Hearing Restoration Project (HRP) is to determine how to regenerate inner ear sensory cells in humans to eventually restore hearing for millions of people worldwide. These sensory cells, called hair cells, in the cochlea detect and turn sound waves into electrical impulses that are sent to the brain. Once hair cells are damaged or die, hearing is impaired, but in most species, hair cells spontaneously regrow and hearing is restored. The HRP is aiming to enable this ability in humans.
All cells develop through a chain of events triggered by chemical signals (proteins) from outside the cell. The signals kick off responses inside the cell that can change the cell’s ability to proliferate (grow and divide) and differentiate (take on specialized functions).
The Wnt signaling pathway, a sequence of events triggered by the Wnt protein, helps guide inner ear cell development, including the proliferation of cells that differentiate into the hair cells and supporting cells necessary for hearing and balance. But in mice and other mammals, inner ear cell proliferation does not continue past newborn stages.
Underscoring their importance in evolutionary terms, Wnt signals occur across species, from fruit flies to humans—the “W” in Wnt refers to “wingless”—and Wnt signaling is guided by dozens of genes. Albert Edge, Ph.D., Alain Dabdoub, Ph.D., and colleagues performed a comprehensive screen of 84 Wnt signaling-related genes and identified 72 that are expressed (turned on) during mouse inner ear development and maturation. Their results appeared in the journal PLoS One this February.
The Wnt signaling network has three primary pathways. Two are known to be integral to the formation of the mammalian inner ear, including the determination of a cell’s “fate,” or what type of cell it ultimately turns into. This is particularly significant because the inner ear’s sensory epithelium tissue is a highly organized structure with specific numbers and types of cells in an exact order. The precise arrangement and number of hair cells and supporting cells is essential for optimal hearing.
The relationship between the Wnt-related genes, the timing of their expression, and the various signaling pathways that act on inner ear cells is extremely complex. For instance, the composition of components inside a cell in addition to the cell’s context (which tissue the cell is in, and the tissue’s stage of development) will influence which pathway Wnt signaling will take. It is known that inhibiting the action of Wnt signaling causes hair cells to fail to differentiate.
The new research complements previous chicken inner ear studies of Wnt-related genes as well as a recent single-cell analysis of the newborn sensory epithelium in mice (conducted by HRP scientist Stefan Heller, Ph.D., and colleagues). Comprehensively detailing these 72 Wnt-related genes in the mouse cochlea across four developmental and postnatal time periods provides a deeper understanding of a critical component of hair cell development, bringing the HRP closer to identifying genes for their potential in hair cell regeneration.
Your Support Is Needed!
Hair cell regeneration is a plausible goal for eventual treatment of hearing and balance disorders.
The question is not if we will regenerate hair cells in humans, but when.
However, we need your support to continue this vital research and find a cure!
Please make your gift today.
Unlocking the Potential for Hair Cell Regeneration
By Laura Friedman
On November 5, 2015, Hearing Health Foundation hosted its second live-video research briefing as part of our effort to provide regular updates on our research programs and progress. Through these briefings, our goal is for our attendees to obtain new information and understanding about hearing loss, prevention and research toward a cure.
Dr. Andy Groves, Hearing Restoration Project consortium member, presented recent research advances and new discoveries, the use of new technology, and our future plans to prevent and cure hearing loss and tinnitus. The HRP was founded in 2011 and is the first and only international research consortium focused on investigating hair cell regeneration as a cure for hearing loss and tinnitus. The overarching principle of the consortium is collaboration: open sharing of data and ideas. The HRP consortium consists of 13 of the top investigators in the audiological space, as well as a scientific director, Dr. Barr-Gillespie.
We wanted to share with you highlights from the presentation, which is available to watch with live captioning or to read with notes summarizing each slide.
Your Support Is Needed!
Hair cell regeneration is a plausible goal for eventual treatment of hearing and balance disorders.
The question is not if we will regenerate hair cells in humans, but when.
However, we need your support to continue this vital research and find a cure! Please make your gift today.
Distilling the Data
By Michael Lovett, Ph.D.
The burgeoning field of bioinformatics allows the Hearing Restoration Project to analyze and compare large genomics datasets and identify the best genes for more testing. This sophisticated data analysis will help speed the way toward a cure for hearing loss and tinnitus.
Since its launch in 2011, the Hearing Restoration Project (HRP) is focused on identifying new therapies that will restore inner ear hair cell function, and hence hearing. Within the consortium, smaller research groups engage in separate projects over the course of the year, to move the science along more quickly.
Over the past decade my group, and the group led by my collaborator Mark Warchol, Ph.D., have worked to identify genes that are potential targets for drug development or for gene therapies to cure hearing loss. Our approach has been to determine the exact mechanisms that some vertebrates—in our case, birds—use to regenerate their hair cells and thus spontaneously restore their hearing. We have been comparing this genetic “tool kit” with the mechanisms that mammals normally use to make hair cells.
Unlike birds, mammals cannot regenerate adult hair cells when they are damaged, which is a leading cause of human hearing and balance disorders. Our working hypothesis is that birds have regeneration mechanisms that mammals are missing—or that mammals have developed a repressive mechanism that prevents hair cell regeneration.
In either case, our strategy has been to get a detailed picture of what transpires during hair cell regeneration in birds by using cutting-edge technologies developed during the Human Genome Project (the international research collaboration whose goal was the complete mapping of all the nuclear DNA in humans). These next-generation (NextGen) DNA sequencing methods have allowed us to accurately measure changes in every single gene as chick sensory hair cells regenerate.
The good news is that this gives us, for the first time, an exquisitely detailed and accurate description of all of the genes that are potential players in the process. The bad news is that this is an enormous amount of information; thousands of genes change over the course of seven days of regeneration.
Some of these will be the crucially important—and possibly game-changing—genes that we want to explore in potential therapies, but most will be downstream effects of those upstream formative events. The challenge is to correctly identify the important causative needles in the haystack of later consequences.
We already know some important genetic players, but we are still far from understanding the genetic wiring of hair cell development or regeneration. For example, after decades of basic research, we know that certain signaling pathways, such as those termed Notch and Wnt, are important in specifying how hair cells develop. These chemical signaling pathways are made of multiple protein molecules, each of which is encoded by a single gene.
However, the Notch and Wnt pathways together comprise fewer than 100 genes and, despite being intensively studied for years, we do not completely understand every nuance of how they fit together.
It also may seem surprising that—more than a decade after the completion of the Human Genome Project and projects sequencing mouse, chick, and many other species’ genomic DNA—we still do not know the exact functions of many of the roughly 20,000 genes, mostly shared, that are found in each organism. This is partly because teasing out all of their interactions and biochemical properties is a painstaking process, and some of the genes exert subtly different effects in different organs. It is also because the genetic wiring diagram in different cells is a lot more complicated than a simple set of “on/off” switches.
All of this sounds a bit dire. Fortunately, we do have some tools for filtering the data deluge into groups of genes that are more likely to be top candidates. The first is to extract all of the information on “known” pathways, such as the Notch and Wnt mentioned earlier. That is relatively trivial and can be accomplished by someone reasonably well versed in Microsoft Excel.
That leaves us with the vast “unknown” world. Analyzing this requires computational, mathematical, and statistical methods that are collectively called bioinformatics. This burgeoning field has been in existence for a couple of decades and covers the computational analysis of very large datasets in all its forms. For example, we routinely use well-established bioinformatic methods to assemble and identify all of the gene sequences from our NextGen DNA sequence reads. These tasks would take many years if done by hand, but a matter of hours by computational methods.
In the case of our hair cell regeneration data, our major bioinformatic task is to identify the best genes for further experimental testing. One method is to computationally search the vast biological literature to see if any of them can be connected into new networks or pathways. There are now numerous software tools for conducting these types of searches. However, this really is not very helpful when searching through several thousand genes at once. The data must be filtered another way to be more useful.
We have used statistical pattern matching tools called self-organizing maps to analyze all of our data across every time point of hair cell regeneration. In this way we can detect genes that show similar patterns of changes and then drill down deeper into whether these genes are connected. This has provided us with an interesting “hit list” of genes that have strong supporting evidence of being good candidates for follow-up.
An additional approach is to compare our chick data to other datasets that the HRP consortium is collecting. The logic here is that we expect key genetic components to be shared across species. For example, we now know a great deal about what genes are used in zebrafish hair cell regeneration and the genes that specify mouse hair cells during normal development. We can conduct computational comparisons across these big datasets to identify what is similar and what is different. Again, this has yielded a small and interesting collection of genes that is being experimentally tested.
Our final strategy has been to extract classes of genes that act as important switches in development. These transcription factors control other genetic circuits. We have identified all of these that change during chick hair cell regeneration. As a consortium the HRP now has a collection of about 200 very good candidate genes for follow-up. However, software and high-speed computation are not going to do it all for us. We still need biologists to ask and answer the important questions and to direct the correct bioinformatics comparisons.
Hair cell regeneration is a plausible goal for the treatment of hearing and balance disorders. The question is not if we will regenerate hair cells in humans, but when. Your financial support will help to ensure we can continue this vital research and find a cure in our lifetime! Please help us accelerate the pace of hearing and balance research and donate today. Your HELP is OUR hope!
If you have any questions about this research or our progress toward a cure for hearing loss and tinnitus, please contact Hearing Health Foundation at info@hhf.org.
Michael Lovett, Ph.D., is a professor at the National Lung & Heart Institute in London and the chair in systems biology at Imperial College London.
Orchestrating Hair Cell Regeneration
By the Stowers Institute for Medical Research
The older we get, the less likely we are to hear well, as our inner ear sensory hair cells succumb to age or injury. Intriguingly, humans are one-upped by fish here. Similar hair cells in a fish sensory system that dots their bodies and forms the lateral line, by which they discern water movement, are readily regenerated if damage or death occurs.
A neuromast sensory structure (green) of the zebrafish lateral line, which helps the fish detect water movement, is shown among surrounding cells (cell nuclei in red).
Credit: Piotrowski Lab, Stowers Institute for Medical Research
A new study in the July 16 online and August 10 print issue of Developmental Cell, from Stowers Institute for Medical Research Associate Investigator Tatjana Piotrowski, Ph.D., zeros in on an important component of this secret weapon in fish: the support cells that surround centrally-located hair cells in each garlic-shaped sensory organ, or neuromast. “We’ve known for some time that fish hair cells regenerate from support cells,” Piotrowski explains, “but it hasn’t been clear if all support cells are capable of this feat, or if subpopulations exist, each with different fates.”
While mammals also have support cells, they unfortunately do not respond to hair cell death in the same way. So understanding how zebrafish support cells respond to hair cell loss may provide insight into how mammalian support cells might be coaxed into regenerating hair cells as well. Zebrafish are particularly amenable to studies of regeneration because transparent embryos and larvae render developmental processes visible and experimentally accessible.
Piotrowski and her team treated zebrafish larvae with the antibiotic neomycin, which kills hair cells, then monitored support cell proliferation in regenerating neuromasts for three days using time-lapse movies. “These single cell lineage analyses were tremendously time-consuming but very informative,” Piotrowski notes. The study’s lead author, Andrés Romero-Carvajal, Ph.D., previously a predoctoral researcher at the Stowers Institute, carefully kept track of every individual support cell’s location and behavior across different time-lapse frames.
The researchers determined that approximately half of the dividing support cells differentiated into hair cells, while the rest self-renewed. Self-renewal is an equally important fate, Piotrowski points out, because it ensures maintenance of a reserve force that, if necessary, can spring into regenerative action. The researchers also observed that lineage fate of support cells hinged on where they were located in the neuromast, as self-renewing cells were found clustered at opposite poles while differentiating cells were distributed in a random, circular pattern close to the center.
Such distinct support cell locations were “strongly indicative of differences in gene expression”, Piotrowski says, so the team turned its attention to exploring some of the genes and signaling pathways involved. A study of gene expression patterns showed that members of the Notch and Wnt pathways were expressed in different parts of the neuromast, specifically the Notch members in the center and the Wnt members at the poles. To determine if and how these two pathways regulate each other, the researchers used an inhibitor to turn off Notch signaling in neuromasts. This halt in Notch activity mimics the halt known to occur immediately after neomycin-induced hair cell death. After inhibitor treatment, they saw transient upregulation of Wnt ligands in the neuromast center, along with support cell proliferation. The majority of the proliferating cells became hair cells.
“We found that Notch directly suppresses differentiation (of support cells into hair cells), and indirectly inhibits proliferation by keeping Wnt in check,” Piotrowski explains. “Previously, others thought perhaps it was Wnt that had to be downregulated, to initiate regeneration. However, our data support the loss of Notch signaling as a more likely trigger.” Essentially, the process of restoring injured or dead hair cells in neuromasts is jump-started by the transient suppression of Notch, while its eventual reactivation restores the balance, ensuring that not all support cells answer the call to regenerate through proliferation and differentiation.
Piotrowski’s research is partially supported by the Hearing Health Foundation through its Hearing Restoration Project (HRP), which emphasizes collaborations across multiple institutions to develop new therapies for hearing loss. By continuing to illuminate the intricacies of hair cell regeneration in zebrafish, she and her team are providing other HRP scientists with candidate genes and molecular pathways to probe in other models such as chicken and mice, with the goal of providing insight that could someday make human inner ear hair cells readily replaceable.
The study was also funded by the Stowers Institute and the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health (award RC1DC010631). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Other Institute contributors include Joaquín Navajas Acedo; Linjia Jiang, Ph.D.; Agnė Kozlovskaja-Gumbrienė; Richard Alexander; and Hua Li, Ph.D.
Lay summary of findings
Hair cells in sensory structures called neuromasts, which form the sensory system fish use to orient themselves in water, are similar to mammalian inner ear hair cells responsible for our sense of hearing. Unlike the latter, however, they are constantly replaced after damage or death. In the current issue of Developmental Cell, Stowers Associate Investigator Tatjana Piotrowski, Ph.D., and members of her lab closely examine, in zebrafish, the support cells from which hair cells regenerate. By tracking individual support cells during neuromast regeneration, first author Andrés Romero-Carvajal, Ph.D., shows that approximately half become hair cells, while the rest self-renew as support cells. These lineage decisions are coordinated by interactions between the Notch and Wnt signaling pathways and are location-specific, as differentiation into hair cells occurs toward the center of neuromasts and self-renewal occurs at opposite poles of the structures. Piotrowski hopes her lab’s findings in zebrafish may be extrapolated to mammals someday, to help provide basic insight needed to progress towards the ultimate goal of regenerating human inner ear hair cells.
About the Stowers Institute for Medical Research
The Stowers Institute for Medical Research is a non-profit, basic biomedical research organization dedicated to improving human health by studying the fundamental processes of life. Jim Stowers, founder of American Century Investments, and his wife, Virginia, opened the Institute in 2000. Since then, the Institute has spent over one billion dollars in pursuit of its mission.
Currently, the Institute is home to almost 550 researchers and support personnel; over 20 independent research programs; and more than a dozen technology-development and core facilities.
The above post is reprinted, with permission, from materials provided by Stowers Institute for Medical Research.
Your financial support will help to ensure we can continue this vital research and find a cure in our lifetime! Please help us accelerate the pace of hearing and balance research and donate today. Your HELP is OUR hope!
If you have any questions about this research or our progress toward a cure for hearing loss and tinnitus, please contact Hearing Health Foundation at info@hhf.org.
The Path to a Cure for Hearing Loss and Tinnitus
By Laura Friedman
On May 21, 2015, Hearing Health Foundation hosted its first live-video research briefing as part of our effort to provide regular updates on our research programs and progress. Through these briefings, our goal is for our attendees to obtain new information and understanding about hearing loss, prevention and research toward a cure.
During this inaugural research briefing, Dr. Peter Barr-Gillespie, Scientific Director, Hearing Restoration Project presented the Hearing Restoration Project (HRP). The HRP was founded in 2011 and is the first and only international research consortium focused on investigating hair cell regeneration as a cure for hearing loss and tinnitus. The overarching principle of the consortium is collaboration: open sharing of data and ideas. The HRP consortium consists of 14 of the top investigators in the audiological space, as well as a scientific director, Dr. Barr-Gillespie.
We wanted to share with you highlights from the presentation, which is available to watch with live captioning or to read with notes summarizing each slide.
History of Hearing Health Foundation
Founded in 1958, established reputation for pioneering breakthroughs in hearing and balance research.
Early supporters of the revolutionary cochlear implant. Today, over 220,000 children and adults benefit.
Advocated for the passage of Universal Newborn Hearing Screening legislation in the 1990s. Today, 97% of newborns are tested for hearing loss at birth.
The Emerging Research Grants Program provides seed funding for researchers in hearing and balance science such as discoveries in hair cell regeneration, tinnitus, hyperacusis, and Ménière’s research.
The Challenge
In the past century, the primary treatment for hearing loss has been hearing aids and cochlear implants. While these have been very successful treatments, they have limitations.
For this century, we have a number of different avenues for more effective therapy.
Preventing the damage to the hair cells to preserve hearing. By generating greater awareness of the effects of hearing loss, we aim encourage people of all ages to protect their ears.
Gene therapy, targeting those who have lost hearing due to genetic disorders.
The majority of people who have lost hearing have done so through noise damage or aging, and may be candidates for hair cell regeneration/restoration.
HRP Consortium History & Model
One of the key facets of the HRP’s approach is that we use three different animal models for studying hair cell regeneration
Two of those models, the chick and the zebrafish, show robust hair cell regeneration.
f you damage the hair cells of a chick or a fish, within a short time—only a day or two for the fish, a few weeks for the chick—the hair cells come back; new hair cells are formed.
So, that's spectacular, because it tells us that animals are capable of regenerating hair cells.
y contrast, the mouse is our other experimental model. Like in the human, the mouse shows no hair cell regeneration after a few days following birth.
You can damage the hair cells in the mouse and as far as we can tell, nothing much happens in terms of restoring hair cells. So, if we can figure out how to regenerate hair cells in the mouse, then we will be able to regenerate hair cells in people.
HRP Strategic Research Plan
Our strategic plan consists of three separate phases. We have already made a lot of progress on Phase 1 and we have initiated Phase 2:
Phase 1 – Discovery research: Compare the fish, chick, and mouse to discover pro- or anti-regeneration pathways and determine supporting cell fates.
Phase 2 – Pathway validation: Verify pathways using fish, chick, and mouse models and describe regeneration strategies.
Phase 3 – Develop therapies and treatment options: Identify drugs that trigger hair cell regeneration in the mouse model.
Progress To-Date
Progress on Phase 1: We've identified a variety of candidates for hair cell regeneration and the pathways that are necessary.
We have too many, so we really are continuing to use bioinformatics methods to winnow down and determine which are most important.
We have definitively shown, at least in the mouse, the specialized supporting cells remain.
We know now what our target cells are for triggering hair cell regeneration.
Phase 2 has begun, but we haven’t stopped Phase 1:
We've got multiple approaches to try and see whether or not we can block regeneration in the fish and chick or stimulate regeneration in the mouse.
Phase 3 is in sight:
Experimental models from Phase 2 will be used to screen for drugs—using the mouse first
The Next Five Years
With your help, we can continue to quicken the pace towards a cure. Here’s our plan for the next five years:
Phase 1 will continue: more candidate generation for Phase 2
Phase 2 (pathway verification) already initiated in zebrafish, mouse, chick (low throughput)
Phase 2 must be scaled up: many more genes, combinatorial approaches; cell lines for screening
Phase 3 (drug screening) requires the right screening model, which will come out of Phase 2.
The Future is Very Bright – But we need your support!
Hair cell regeneration is a plausible goal for eventual treatment of hearing and balance disorders. The question is not if we will regenerate hair cells in humans, but when. However, we need your support to continue this vital research and find a cure! Please make your gift today.
The Danger From Noise When It Is Actually Music
By Yishane Lee
Les Paul Ambassador
John Colianni
Noise-induced hearing loss affects anyone exposed to very loud or chronic noise. It doesn’t matter if the “noise” is actually music. It has been estimated that up to half of classical orchestral musicians have hearing loss because of their work in music, practicing or performing up to eight hours a day. Sound levels onstage, no matter the music genre, can reach up to 110 decibels (dB), although it is not usually continuous. That is equivalent to a jackhammer—even if there’s a melody behind it.
Researchers at the Nofer Institute of Occupational Medicine in Poland measured the exposure for classical musicians as 81 to 90 dBA (A-weighted decibels, a unit of measure for how humans perceive sound) for 20 to 45 hours a week. In their study published in the International Journal of Occupational Safety and Ergonomics, they estimated that this exposure over the course of a career increases the risk of a hearing loss of 35 dB by 26 percent. At the greatest risk for hearing loss are those in the brass section—horn, trumpet, tuba—as well as those playing percussion, the study found.
Prolonged exposure at 85 dB (the sound of heavy traffic) will permanently damage the delicate hair cells of the inner ear, leading to hearing loss. Tinnitus, or ringing in the ears, is another potential problem. Roughly 90 percent of tinnitus cases occur with an underlying hearing loss.
Not surprisingly, rock and jazz musicians are not immune. Indeed, there are a number of well-known rock and pop musicians who have publicly discussed their hearing loss and/or tinnitus, among them Sting, Eric Clapton, Neil Young, Phil Collins, and Will.i.am.
But hearing loss due to noise (or music) is completely preventable. A related study by the Polish scientists determined that brass players benefitted the most from the use of custom-molded, silicone earplugs with acoustic filters that reduced sound levels. Woodwind, percussion, and string players also benefited.
In 2013, the Les Paul Foundation and HHF teamed up to launch the Les Paul Ambassadors program. Guitar great Les Paul was determined to find a cure for hearing loss and tinnitus, and through his foundation’s support of HHF’s Hearing Restoration Project, an international research consortium of top hearing scientists, we have the opportunity to find a cure. Learn about the program and the first Ambassador, Lou Pallo, as well as our other Ambassadors saxophonist Chris Potter and jazz pianist John Colianni.
Learn more about NIHL and its risk factors, treatment, and prevention in our new Summer issue of Hearing Health magazine.
What Does a Chicken Have to Do with Hearing Loss?
http://aubankaitis.com/2014/05/14/chicken-and-hearing-loss/
What would you think if someone told you that a baby chick holds the cure for hearing loss? One of the keys to restoring normal hearing in humans is cochlear hair cell regeneration, something that most animals other than mammals, including chickens, can do. The Hearing Health Foundation recently launched a new public service announcement (PSA) called “Chirp the News” which features a baby chick with hearing loss who goes on to live a happy, normal-hearing life. After viewing it, my curiosity was piqued. I had an opportunity to ask Shari Eberts, Chairman of the HHF’s Board of Directors, a couple of questions and wanted to share what I learned.
Question: For those that are not familiar with your organization, what is the Hearing Health Foundation and/or what is the Foundation’s mission?
Hearing Health Foundation (HHF) is the largest private funder of hearing research, with a mission to prevent and cure hearing loss and tinnitus through groundbreaking research. Since 1958 HHF has given away millions of dollars to hearing and balance research, including work that led to cochlear implant technology and now through the Hearing Restoration Project is working on a cure for hearing loss and tinnitus. Hearing Health Foundation also publishes Hearing Health magazine, a free consumer resource on hearing loss and related technology, research, and products.
Question: Shari, it is my understanding that you acquired a hearing loss in your late 20′s. Can you tell me a little bit about how your hearing loss was identified, the cause of your hearing loss, and how it has impacted your personal and professional lives?
I first noticed my hearing loss in business school. Students were participating in class, and I would sometimes miss their comments, particularly the funny ones that were made almost as an aside. My father and my grandmother both had a hearing loss, so I figured I should get tested. It turns out that I had a mild hearing loss in both ears. The loss is genetic and is centered in the mid-range or speech frequencies. Luckily, my high pitch hearing is almost perfect. My loss has gotten progressively worse each year since business school, but I am able to manage it with hearing aids and by advocating for myself. At first, I didn’t want to admit that I had a hearing loss, and I hid it from others, but eventually I began to realize how much better my life could be if I used my hearing aids, and I began wearing them all the time. I am glad that I do.
As someone who lives with hearing loss everyday, I am personally thrilled with the prospects for a cure. Life with hearing loss can be frustrating. Sometimes you miss the joke when everyone else is laughing and sometimes you miss important information because you don’t hear it. Supportive family and friends can make living with hearing loss easier, but a genuine cure would be life changing. After having met and worked with our consortium scientists for these past two years, I am confident that we will have a cure in my lifetime. I am counting the days.
Question: Knowing that you acquired a hearing loss in your late 20′s, it makes sense that you would be passionate about educating people about hearing loss and learning about various research focusing on a cure. With so many different organizations dedicated to hearing loss, what made you specifically gravitate toward Hearing Health Foundation? What makes this organization so unique?
HHF’s approach to research is unique and I believe it will shorten the timeline to a cure. For years, scientific research has been conducted in relative isolation—one researcher or one institution working alone to tackle a major health issue. HHF developed the HRP Consortium model to do things differently. Our HRP scientists work on research projects together, share their unpublished data and tools, and collaborate on the development and refinement of the HRP’s strategic research plan. The group meets bi-annually in person, monthly by conference call, and communicates frequently by email. This continual dialogue is helping to eliminate repetitive work across the team, saving time and research dollars, and most importantly, accelerating the timetable to a cure.
Our HRP Consortium is the dream team of hair cell regeneration, comprising the best auditory scientists at leading institutions worldwide such as Harvard and Stanford. With more than 200 years of combined experience in hearing research, the HRP Consortium publishes widely (over 400 published papers among them) and have well established labs (receiving over 600 NIH grants combined). We have every confidence we have the right team in place, and the right model to accelerate the timeline to a cure.
Question: The Hearing Health Foundation was established in 1958 and had been seeking donations from the public to help fund “groundbreaking research” for the prevention of and cure for hearing loss. Can you provide a historical synopsis of some of the more significant research achieved by the Foundation since its inception?
HHF’s founder, Collette Ramsey Baker, was steadfast in her support of funding for new technologies and treatments for hearing loss. For example, back in the 1960s, HHF began funding research into cochlear implant technology. HHF’s founder, Collette Ramsey Baker, prevailed despite objections and doubts from supporters that she was wasting money. Cochlear implants have proven to be a valuable treatment option for people with profound hearing loss, benefiting 125,000 people in the U.S. and 300,000 people worldwide. HHF has also research that led to the development of many of today’s standard treatments for otosclerosis (abnormal bone growth in the ear) and ear infections. In the 1990s, HHF was a leader in advocating for Universal Newborn Hearing Screening legislation, which increased testing from 5% of newborns to 94% by 2007. In 2011, HHF launched our most important project yet, the Hearing Restoration Project (HRP), which aims to discover a biological cure for hearing loss and tinnitus.
Question: What research is the Foundation currently working on that is anticipated to have a significant and/or practical impact on hearing loss prevention and/or cure within the next 10 years?
HHF officially launched its Hearing Restoration Project (HRP) in 2011 and is currently funding 5 projects from its consortium scientists, but the initial discovery that led to the HRP came many years before. Many types of hearing loss result from damage to the delicate hair cells of the inner ear. Humans can’t regrow these cells—but in a game-changing breakthrough in 1987, HHF-funded scientists discovered that birds can. While studying how drugs that are known to cause hearing damage affect the tiny sensory cells in the ear, these scientists needed to permanently damage a chicken’s hair cells. For 10 days, research assistants administered a common antibiotic, known to cause hearing loss, to laboratory chickens. On day 11 many of the hair cells were lost and a few days later, even more were lost. Surprisingly, when the scientists looked three weeks later, almost all the hair cells had returned. They didn’t believe these results so they did the experiment again and again. Sure enough, chickens can naturally regenerate their inner ear hair cells, restoring their hearing after damage.
The amazing thing is that regeneration happens naturally and very robustly in almost all animals – mammals are the exception. This makes HHF and the researchers confident that we will find a way to stimulate this regeneration in mammals, including humans.
The HRP consortium of scientists has developed a strategic research plan to develop a cure for hearing loss and tinnitus in 10 years. This three-phase plan starts with discovery research and culminates in clinical trials. The plan, developed specifically by the HRP scientists and updated to incorporate new findings and approaches, is a living document meant to guide but not limit the work. Relevance to this strategic plan is one of the criteria for a project to receive HRP funding.
The HRP is currently in Phase I of its strategic research plan (years 1-5). This first phase focuses on searching for the genes or series of genes that trigger natural regeneration of hair cells in animals such as birds and zebrafish. This phase will also examine which genes in mammals prevent the natural regeneration of hair cells. Finally, Phase I will determine the types of cells in mammals’ ears that could serve as available targets for regeneration therapies. Phase II (years 3-8) starts with the residual cells that remain in a mammal’s inner ear after hearing loss and uses the genes identified in Phase I to trigger hair cell regeneration. In Phase III (years 8-10), the HRP Consortium will partner with a pharmaceutical or other company to develop drugs that mimic the identified genes, resulting in a regenerative therapy.
Question: How can audiologists and other hearing health care providers get involved with the Hearing Health Foundation?
HHF is always eager to partner with hearing health care providers! In fact, we have developed a brochure specifically for use by hearing health care providers that includes important information for their patients about how hearing works, the types of hearing loss, and common treatment solutions. It also lets patients know about the resources HHF can offer, like its free quarterly magazine. Hearing Health Magazine is the award-winning leading consumer publication on hearing loss filled with the latest on research breakthroughs, strategies to manage hearing loss, personal stories, hearing technologies and products and features on seniors, pediatrics, parents, musicians, veterans and more! Please feel free to contact us at info@hhf.org if you are a hearing health care professional and would like copies of our patient brochure or magazine.
Question: How can the general public support the mission and goals of the Hearing Health Foundation?
There are lots of ways for people to learn more about HHF and help support our research for a cure for hearing loss and tinnitus.
Visit our website to learn more
Stay up to date on all the latest news by liking us on Facebook and following us on Twitter
Sign up for our informative monthly e-newsletter
Subscribe to Hearing Health Magazine, our award-winning leading consumer publication on hearing loss. Get the latest on research breakthroughs, strategies to manage hearing loss, personal stories, hearing technologies and products, and features on seniors, pediatrics, veterans, musicians and more.
Inspire others by sharing your personal story and draw comfort from the stories of others
Make a tribute gift to honor a loved one with hearing loss or a favorite audiologist
Support our work with a tax-deductible donation
Shari Eberts is Chairman of the Board of Directors at the Hearing Health Foundation, an organization whose mission is to prevent and cure hearing loss and tinnitus via collaborative, groundbreaking research. She received her BS from Duke University in 1990 and MBA from the Harvard Business School in 1995. Previously employed by Goldman Sachs and McKinsey & Company, Shari spent 13 years at J.P. Morgan in the capacity of a senior equity analyst (broadlines retail) and, most recently as Associate Director of U.S. Equity Research. This mom of two and former Wall-Streeter joined HHR in 2010 and has committed herself to supporting the search for a cure for hearing loss and tinnitus.